AG Prof. Dr. Elke Ober
The group of Professor Elke Ober is interested in the dynamic cell behaviours and cell-cell interactions assembling the functional architecture of the liver in development and its reestablishment during regeneration.
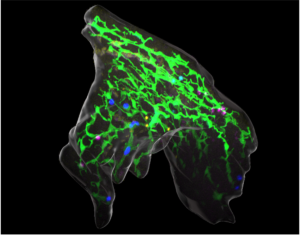
The liver performs a plethora of metabolic functions, which are essential for body homeostasis and depend on its specialized tissue architecture. Chronic liver damage can lead to scaring and loss of tissue integrity, whereas uncontrolled tissue remodeling can transition into liver cancer. Detailed knowledge of the livers 3D cellular organization, its development and plasticity is therefore required. In the embryo, progenitor cells differentiate into hepatocytes and biliary cells, which together with blood vessels and additional cell types set up the complex tissue morphology of the liver. Despite its medical relevance, it is poorly understood how hepatocytes connect to biliary ducts, align along blood vessels and generally how the specialized liver architecture is established in the embryo, maintained in homeostasis and repaired after injury. Our research therefore aims to identify the molecular and cellular mechanism directing liver cell-cell communication, movement and remodeling essential for directing tissue morphogenesis in the embryo and during regeneration.
We use zebrafish as a model, which is ideal for studying tissue morphogenesis, because embryos and larvae are transparent and cell behaviours can be visualized at single cell resolution in the living animal. For this, we have generated new transgenic tools that enable the monitoring and tracking of individual cells in 4D and by combining them with different genetic, cell biological, quantitative imaging and biophysics approaches allow to analyse liver morphogenesis and the establishment of the specialized tissue architecture.
Ongoing projects
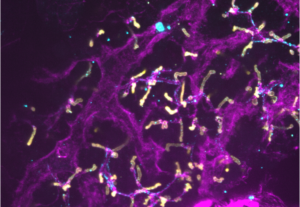
Liver differentiation is spatially organised and occurs in 3D, with hepatocytes aligning basally along blood vessels and connecting via their apical domain to the biliary network, giving rise to functional units. Current models, including in vitro differentiation of hPSC-derived hepatocytes and organoids are incomplete, emphasizing the need for understanding the 3D cellular and mechanical environment and their cues driving organ architecture maturation in vivo.
How is the integration of new cells into remnant tissue regulated in regeneration? Chronic liver damage can lead to scaring and loss of tissue integrity, compromising physiologic functions and the innate ability of the liver to heal. Research on liver regeneration has made great progress in understanding the restoration of tissue mass. We have developed a light-inducible hepatocyte ablation tool, which separates the injury and repair phase and combined with light-sheet microscopy enables monitoring of restorative cell behaviours (Ambrosio, Bailey, et al., 2024). Employing our new optogenetic ablation tool and advanced imaging technologies, we will build a quantitative 4D-model of tissue repair that integrates the contributions of cell interactions, migration and generally tissue remodeling.
- Ambrosio, E.M.G.*, Bailey, C.S.L.*, Unterweger, I.A., Christensen, J.B., Bruchez, M.P., Lundegaard, P.R. and Ober, E.A. (2024) LiverZap: A chemoptogenetic tool for global and locally restricted hepatocyte ablation to study cellular behaviours of liver regeneration; Development 151 (4): dev202217. doi.org/10.1242/dev.202217.
- Unterweger, I.A. Klepstad, J., Hannezo, E., Lundegaard, P. R., Trusina, A. and Ober, E.A. (2023) Lineage tracing identifies heterogenous hepatoblast contribution to cell lineages and organ growth dynamics PLoS Biol. 4;21(10):e3002315. doi: 10.1371/journal.pbio.3002315
- Caviglia S*, Unterweger I.A.*, Gasiunaite A,. Vanoosthuyse, A.E., Cutrale, F., Trinh, L.A., Fraser S.E., Neuhauss S.C.F., and Ober E.A.. (2022) FRaeppli, a multispectral imaging toolbox for lineage tracing and dense tissue analysis. Development 149(16):dev199615. doi: 10.1242/dev.199615
- Thestrup, M. I., Caviglia,, , Cayuso, J., Heyne, R. S. L., Satriano, L., Ahmad, R., Hofmeister, W., Andersen, J. B., Wilkinson, D. G. and Ober, E. A. (2019) A morphogenetic EphB/EphrinB code controls hepatopancreatic duct formation. Nature Communications 10(1):5220; doi: 10.1038/s41467-019-13149-7
- Cayuso, J., Dzementsei, A., Fischer, J. C., Karemore, G., Caviglia, S., Bartholdson, J., Wright, G. J. & Ober E. A.EphrinB1/EphB3b coordinate bidirectional epithelial-mesenchymal interactions controlling liver morphogenesis and laterality. Dev Cell. 39, 316-328 (2016).